Electric dipole
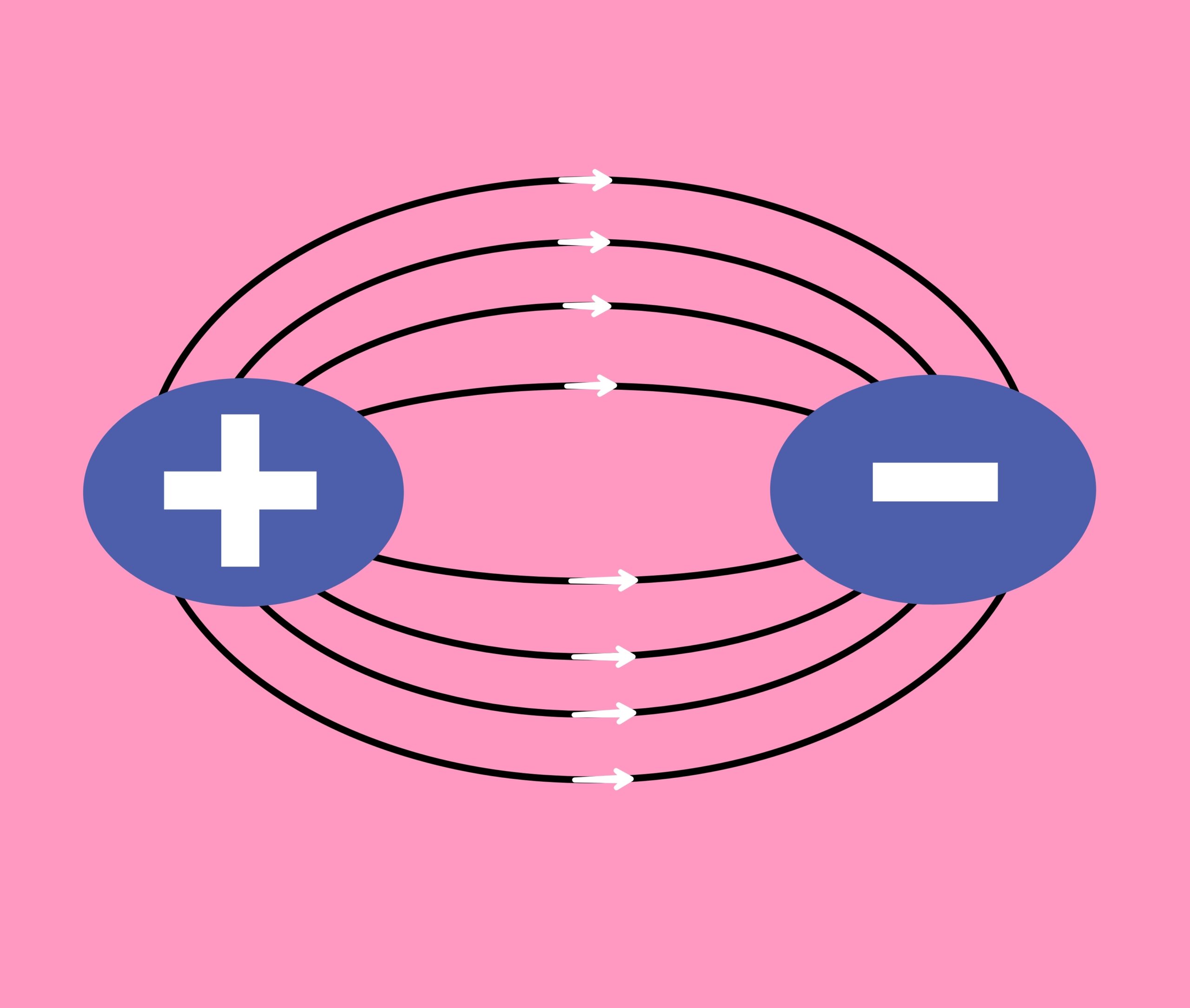
Electric dipole is a system in which two equal but opposite type of point charges are located at a short distance from each other.
The product of any one charge and the short distance between the two charges is called electric dipole moment.
It is represented by P.
P= q.2l cm (meter . Coulomb)
Where
p= electric dipole
q= Either charge of dipole
2l= Dipole length
The moment of electric dipole is a vector quantity. Whose direction is from negative charge to positive charge along the axis of the dipole.
Dimension of electric dipole moment
P = 2ql
p = [ATL]
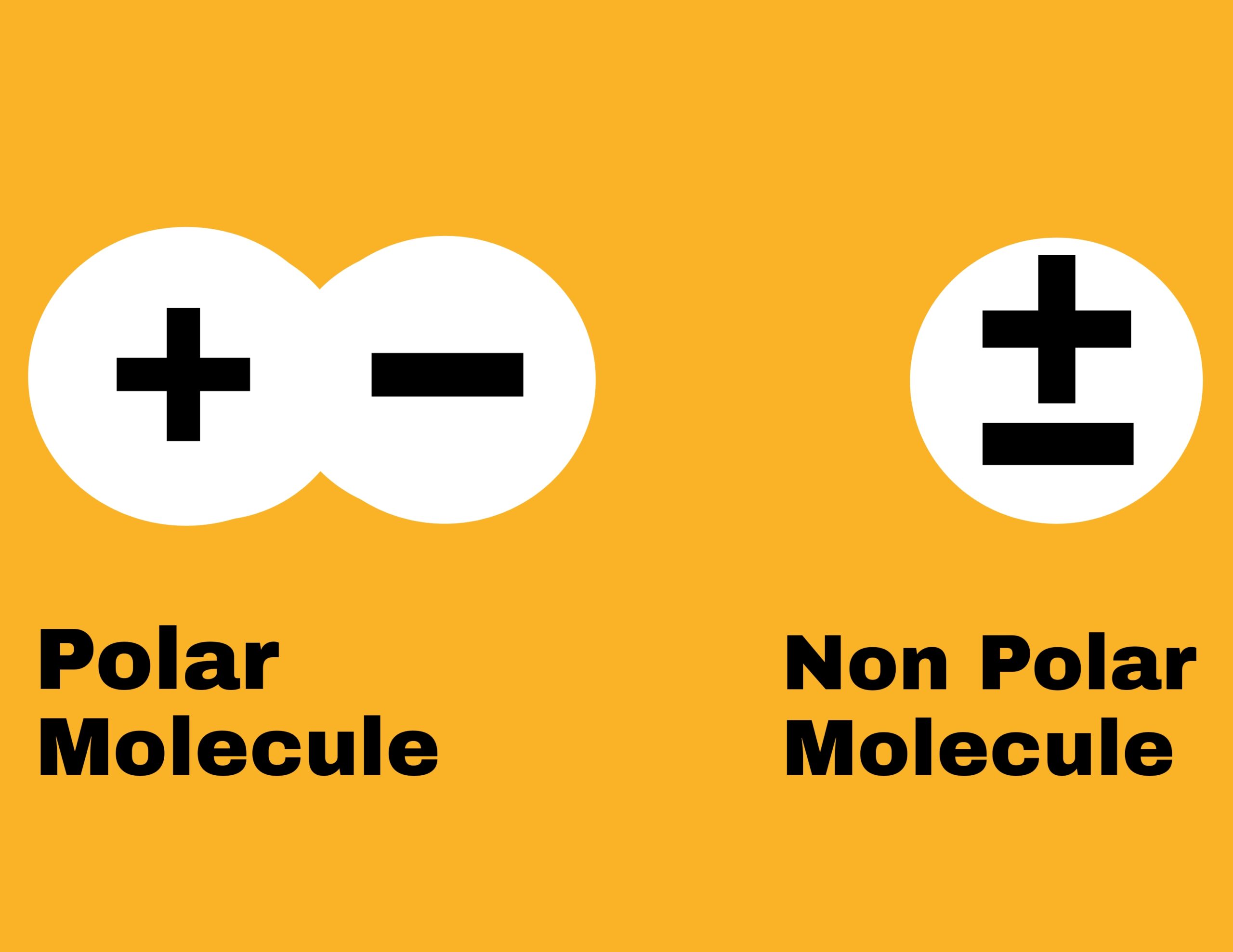
Polar molecules
The substance which is made up of such molecules whose centers of positive and negative charge are different, then the substance made up of such molecules is called polar substance and the molecules are called polar molecules. These molecules are responsible for the flow of electric current in the substance.
Example
HCl, NaCl etc.
Non polar molecules
Such molecules in which the concentration of positive and negative charge is at same point i.e. there is no space between positive and negative charge.
The substance made up of non-polar molecules is called non-polar molecules. These substances are bad conductors of electricity.
Example
H₂, N₂, H₂O etc.